未明原因的pFSGS
近來研究顯示,抗-Nephrin自身抗體(Anti-nephrin Ab)在微小病變(MCD)和局灶性節(jié)段性腎小球硬化(FSGS)的發(fā)生中扮演重要的作用,本文概述了抗-Nephrin抗體作為MCD和原發(fā)性FSGS(pFSGS)的血液循環(huán)滲透因子的研究進展,并對將來的研究方向提出了展望。
MCD和FSGS病因主要分三類:足細胞相關(guān)基因遺傳突變引起、感染或藥物使用等繼發(fā)性因素和不明原因的原發(fā)性因素造成。遺傳突變只占比在8~14%左右[1-3],繼發(fā)性因素占比20-30%[4-5],剩下的基本都是由不明原發(fā)性因素引起。
循環(huán)因子理論與FSGS
當(dāng)1972年Hoyer等[6]第一次報道了腎移植術(shù)后復(fù)發(fā)FSGS的患者開始,研究人員就推測血液中某種循環(huán)滲透因子可能引起了FSGS的產(chǎn)生。隨之流行病學(xué)研究顯示,移植后FSGS具有較高的復(fù)發(fā)率,有時在移植后幾小時內(nèi)就會發(fā)生,這結(jié)果進一步促使研究人員相信,致病因子可能不是“腎臟內(nèi)的局部現(xiàn)象”,而是一種血液循環(huán)滲透因子,它通過改變腎小球屏障來損傷足細胞[7-8]。直到2012年Gallon等[9]在一個FSGS復(fù)發(fā)的患者案例研究中發(fā)現(xiàn),當(dāng)移植腎被切除并再次移植給另一個沒有FSGS病史的受者時,腎臟的功能得到了恢復(fù),且FSGS特有的組織病理學(xué)損害也發(fā)生了逆轉(zhuǎn),這基本確定了是血液中存在的某些循環(huán)滲透因子導(dǎo)致了FSGS的發(fā)生。
隨后幾年,大量的研究資源投入到尋找在MCD和pFSGS蛋白尿形成中起關(guān)鍵作用的循環(huán)滲透因子[10-11]。盡管投入了非常大的資源和研究努力,這些未知的循環(huán)通透性因子的鑒定一直是艱苦漫長的,研究者并沒有找到可靠一致的生物標志物。
一些被認為增加血清蛋白血管透過性的候選生物標志物分子(表1),包括可溶性尿激酶型纖溶酶原激活物受體(suPAR)[12]、心肌營養(yǎng)素樣細胞因子-1 (CLCF-1)[13]、可溶性CD40配體[14]等等,已經(jīng)發(fā)現(xiàn)與MCD和pFSGS的進展和復(fù)發(fā)相關(guān),但是驗證其臨床效用的研究表明,這些標記物并不在MCD和FSGS的發(fā)展中起到?jīng)Q定性作用,它們也可以在健康人和非腎病綜合征患者血清中檢測到[15-17]。
表1. FSGS和MCD相關(guān)循環(huán)滲透因子及其局限性
Nephrin蛋白介紹
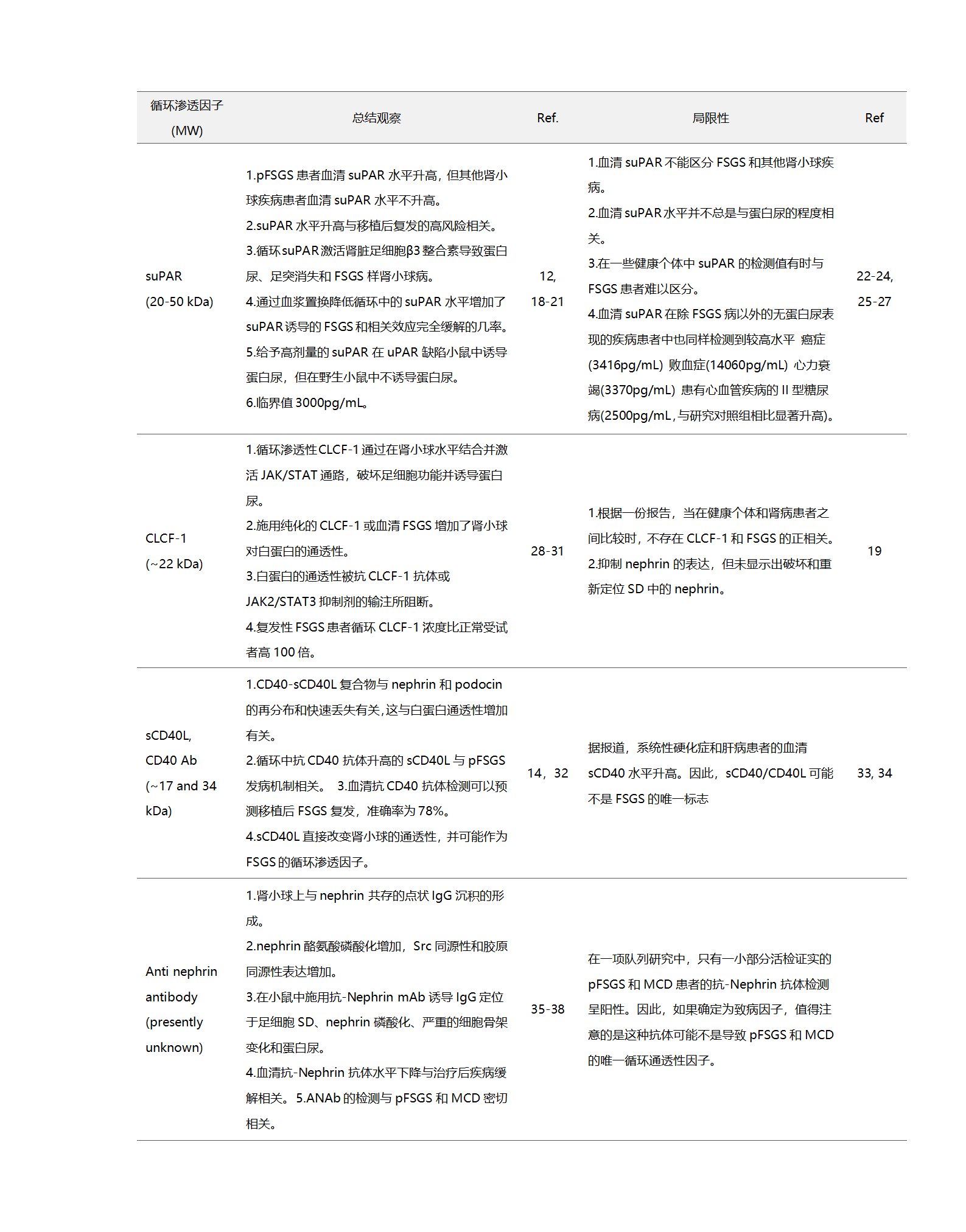
1966年,在一組患有先天性腎病綜合征(NPHS1)[39]的芬蘭家系中,研究發(fā)現(xiàn)這是一種罕見的遺傳基因突變造成的腎臟疾病,但導(dǎo)致這種疾病的具體基因未知。直到1998年NPHS1基因所在的關(guān)鍵染色體區(qū)域被鑒定和測序, NPHS1基因的表達產(chǎn)物被命名為Nephrin,其基因座定位于染色體19q12-q13.1[40-41]。雖然Nephrin蛋白的精確結(jié)構(gòu)和功能在此時仍然未知,但據(jù)推測,其結(jié)構(gòu)域結(jié)構(gòu)類似于屬于免疫球蛋白家族的一大類細胞粘附受體,它可以作為粘附受體和信號蛋白[40]發(fā)揮作用。在后續(xù)研究中[42-43],證實Nephrin是形成足細胞裂孔隔膜相關(guān)復(fù)合物(slit diaphragm, SD)的組成部分,其與腎小球內(nèi)皮細胞和基底膜(GBM)一起形成腎小球濾過屏障。(圖1)
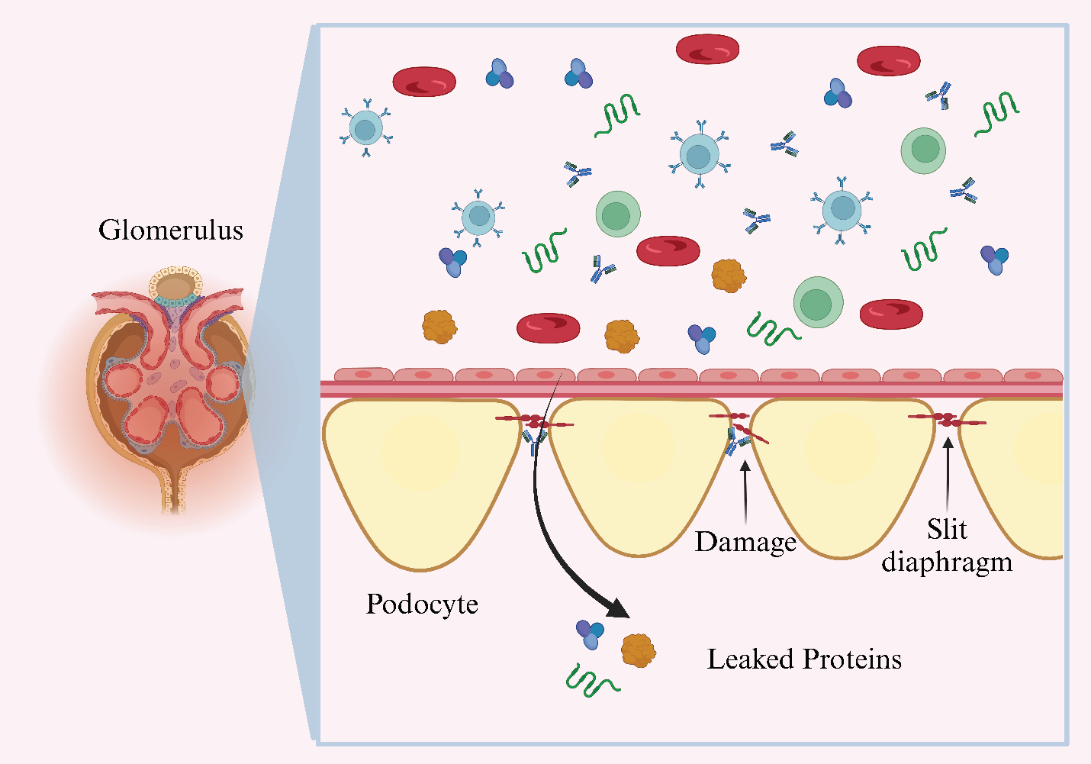
圖1. Nephrin抗體誘導(dǎo)縱膈改變、足細胞損傷和蛋白質(zhì)滲漏入尿液中
Nephrin是一種跨膜蛋白,其N-末端為胞外片段,C-末端為胞內(nèi)結(jié)構(gòu)域,使用對人Nephrin的N-末端特異的抗體檢測證實了其位于腎的縱膈[44]。整個蛋白由八個免疫球蛋白樣胞外結(jié)構(gòu)域、一個纖連蛋白III型樣結(jié)構(gòu)域、一個跨膜結(jié)構(gòu)域和一個短的胞內(nèi)結(jié)構(gòu)域組成,它通過與肌動蛋白細胞骨架[40,45]的相互作用而維持足細胞的組織形態(tài)結(jié)構(gòu)和功能。作為免疫球蛋白超家族的細胞表面受體蛋白,Nephrin還參與細胞間粘附和信號傳導(dǎo)功能。當(dāng)Src激酶家族的Fyn磷酸化Nephrin的胞內(nèi)結(jié)構(gòu)域的六個酪氨酸殘基(Tyr1114、Tyr1136、Tyr1176、Tyr1183、Tyr1193、Tyr1217)中的一個或多個時,由Nephrin的胞內(nèi)尾部介導(dǎo)的下游信號傳導(dǎo)被激活[46]。具體表現(xiàn)為磷酸化的胞內(nèi)結(jié)構(gòu)域會與幾種足細胞胞質(zhì)蛋白相互作用,包括podocin、CD2AP、NEPH1和磷脂酰肌醇3-激酶(PI3K),以將下游信號傳遞到肌動蛋白細胞骨架,從而調(diào)節(jié)足細胞的結(jié)構(gòu)完整性和腎小球濾過狹縫的功能[47-48]。磷酸化Nephrin促進Nck銜接蛋白、ZO-1、連環(huán)蛋白、桶蛋白、足細胞素、CD2-AP和PI3K等的募集并與之相互作用,Nck銜接蛋白發(fā)出肌動蛋白重塑的信號,并參與多種細胞內(nèi)信號通路的調(diào)節(jié),從而影響對足細胞穩(wěn)定至關(guān)重要的肌動蛋白的聚合動力學(xué)[48-49](圖2)。
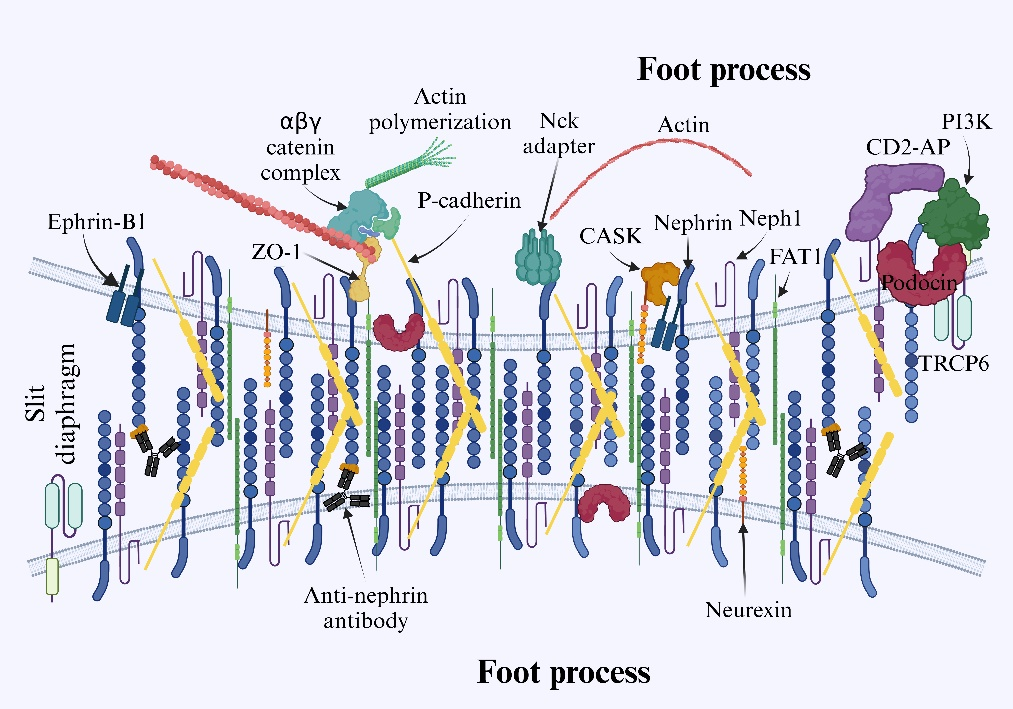
圖2. 相鄰足突之間的縫隙層示意圖。
腎病綜合征與Nephrin抗體
在對NPHS1基因(Nephrin)進行鑒定和測序進行研究之前,科學(xué)家已經(jīng)開始對能夠結(jié)合到腎小球足突表面的抗體及其誘導(dǎo)蛋白尿的特征進行了探索。Orikasa等人[50]用膠原蛋白酶處理過的Wistar大鼠腎小球免疫BALB/b小鼠,產(chǎn)生了高度器官特異性和物種特異性的抗體IgG1,稱為mAb 5-1-6。在體外研究中,觀察到mAb 5-1-6結(jié)合到腎小球足突的表面。當(dāng)在大鼠中注射該mAb時,立即誘導(dǎo)蛋白尿,不需要補體激活。**這項開拓性的研究是建立循環(huán)滲透因子抗體和腎病綜合征蛋白尿誘導(dǎo)之間強關(guān)聯(lián)的證據(jù)。**該方法后來被研究人員用于在動物模型中產(chǎn)生類似的Nephrin抗體,以研究腎小球內(nèi)的Nephrin定位,并作為誘導(dǎo)蛋白尿和腎病狀況的潛在藥物靶點[42-43,51]。
雖然動物模型已經(jīng)成功地證明了抗-Nephrin抗體在介導(dǎo)足細胞損傷中的作用,但在過去幾年中,研究其在腎病綜合征患者中的作用的報道仍然很少。Watts等人[37]最近進行的一項多中心隊列研究,重新激發(fā)了人們對重新評估抗-Nephrin抗體和腎病綜合征發(fā)展之間關(guān)系的興趣。在研究招募的MCD患者中,18例(29%)抗-Nephrin抗體檢測陽性,44例(71%)檢測陰性。活檢分析顯示Nephrin所在的足細胞SD區(qū)域與點狀的IgG沉積共定位。在一些IgG沉積的活組織檢查中,出現(xiàn)一種Nephrin的重新分布遠離SD的現(xiàn)象,表明抗體對Nephrin定位和破壞的影響。當(dāng)MCD活動期患者接受治療時,高水平的循環(huán)抗-Nephrin抗體顯著減少或消失,這與治療期顯著的蛋白尿減少相關(guān)。Hengel等人[36]最近的一項研究顯示,在539名被診斷為腎小球疾病的患者中,105名成人MCD患者中有46名(44%),74名FSGS患者中有7名(9%)都具有較高的抗-Nephrin抗體水平。此外,在被診斷為特發(fā)性腎病綜合征的兒童隊列中,182名中有94名(占52%)表現(xiàn)出較高水平的抗-Nephrin抗體。**在未接受免疫抑制治療的患者中發(fā)現(xiàn)MCD和特發(fā)性腎病綜合征患者的抗體陽性率更是高達69%和90%。**在研究受試者中,與抗體檢測陰性的患者相比,陽性的MCD和FSGS患者表現(xiàn)出更嚴重的腎病綜合征。在小鼠模型中,觀察到IgG沉積在足細胞的SD區(qū),特別是在出現(xiàn)足突融合區(qū)域沉積更明顯。Shirai等人[38]同樣報道了這一觀察結(jié)果。在隨訪期間,觀察到接受糖皮質(zhì)激素和環(huán)孢素免疫抑制治療的患者表現(xiàn)出短暫的緩解,而接受利妥昔單抗(CD20)治療的患者表現(xiàn)出抗體水平完全和持續(xù)的緩解[52]。為了檢測抗-Nephrin抗體對縱裂膈膜的直接作用,對注射抗體的小鼠在3周時的磷酸化蛋白質(zhì)組分析顯示Nephrin在酪氨酸殘基Y1191處磷酸化增加。這個酪氨酸殘基的磷酸化可能導(dǎo)致肌動蛋白裝配、細胞骨架重組和Nephrin內(nèi)吞。
在Cui和Zhao[53]關(guān)于Hengel等人[36]研究的綜述報告中,提出這些抗-Nephrin自身抗體可能不僅限于IgG類,在MCD和FSGS中也可觀察到其他類如IgM的沉積。盡管Shirai等人[38]在復(fù)發(fā)期間的一些活組織檢查中觀察到痕量IgM和C3的沉積,但這表明這些沉積并沒有與Nephrin共定位。此外,在緩解后沒有觀察到IgG沉積的跡象,這表明循環(huán)抗-Nephrin抗體高度可能是一種循環(huán)通透性因子,與腎移植后復(fù)發(fā)的發(fā)病機制有關(guān)。在Bressendorff等人[52]最近報告的一個病例中,一名84歲的男性患者因呼吸急促和水腫入院,他有多種疾病史,包括3期慢性腎病,經(jīng)治療后出院。患者再次入院時,經(jīng)尿檢發(fā)現(xiàn)出現(xiàn)進行性急性腎損傷,伴有低白蛋白血癥和蛋白尿。腎活檢顯示FSGS發(fā)展為與Nephrin重疊的點狀I(lǐng)gG沉積,伴有足突消失,但未觀察到IgM、IgA、C3、C1q或κ和λ輕鏈的形成,患者對糖皮質(zhì)激素治療無反應(yīng)。檢測顯示循環(huán)抗-Nephrin抗體陽性,該抗體在血漿置換治療后逐漸下降。經(jīng)過七次血漿置換治療后,抗體水平低于檢測限,與對照人群中報告的水平相似,同時基線腎功能恢復(fù),腎功能保持穩(wěn)定一年半,無復(fù)發(fā)跡象。
腎移植FSGS復(fù)發(fā)與Nephrin抗體
在Shirai等人[38]進行的一項多中心研究中,在22例FSGS腎移植受者(8例為基因突變致病和14例為非遺傳性患者)中,14例非遺傳性FSGS患者中有11例移植后出現(xiàn)FSGS復(fù)發(fā),與非復(fù)發(fā)FSGS(165 U/mL)和遺傳性FSGS(113 U/mL)患者相比,11例復(fù)發(fā)患者的抗-Nephrin抗體水平顯著升高(移植前和移植后復(fù)發(fā)時分別為831U/mL和1292 U/mL)。抗體水平的升高與提示出現(xiàn)了FSGS的復(fù)發(fā),抗-Nephrin抗體水平也與移植后患者的蛋白尿水平相關(guān),在復(fù)發(fā)性FSGS患者中觀察到更高水平的蛋白尿。最近Batal I等[54]評估了腎移植術(shù)前的抗-Nephrin抗體水平對術(shù)后彌漫性足細胞病(DP)復(fù)發(fā)的預(yù)測價值,回顧性分析了38例移植前留存有血清樣本的患者,在中位隨訪43個月(四分位距8-79個月)后,21例患者出現(xiàn)DP復(fù)發(fā),17例無DP復(fù)發(fā)。移植前抗-Nephrin抗體水平能夠預(yù)測疾病復(fù)發(fā)(曲線下面積0.78,P=0.03)。當(dāng)用之前研究的187U/ml作為閾值診斷時,21例復(fù)發(fā)患者中有8例(38%)為抗體陽性,17例無復(fù)發(fā)患者中全部為抗體陰性(P=0.005)。抗-Nephrin抗體水平預(yù)測DP具有100%的特異性、100%的陽性預(yù)測值、38%的敏感性和57%的陰性預(yù)測值。**提示術(shù)前高水平的抗-Nephrin抗體患者,術(shù)后要密切關(guān)注腎病的復(fù)發(fā)(本隊列顯示100%復(fù)發(fā)),需程序性監(jiān)測抗-Nephrin抗體水平。**生存分析(time-to-event analysis)結(jié)果顯示,移植前抗-Nephrin抗體陽性的患者DP復(fù)發(fā)風(fēng)險更高(風(fēng)險比4.9,95%置信區(qū)間1.25-18.8,P<0.001)。
展望:機遇和挑戰(zhàn)
與其他血液循環(huán)滲透因子(suPAR、CD40等)不同,目前還沒有研究否定抗-Nephrin抗體作為FSGS和MCD的潛在標志物和血液循環(huán)滲透因子的臨床價值。但是已報道的研究有一定的局限性,未來可能需要考慮和解決。
(1)超靈敏、標準化定量分析方法對于確保抗-Nephrin抗體檢測準確和可比性非常重要。目前主要限制因素是缺乏商業(yè)化的人抗-Nephrin抗體,且抗體是多克隆還是單克隆未知,不同患者間是否一致也未知。因此,目前依賴于用陽性的患者血清來制備標準曲線,這會導(dǎo)致不同實驗室的閾值不一致。例如,在Watts等人[37]的研究中,對于1:100的樣品稀釋度,健康人的最大抗-Nephrin抗體閾值設(shè)定為187 U/mL。在Shirai等人[38]的研究中,在1:400的樣本稀釋度下,抗-Nephrin抗體陽性閾值被定義為最大抗體滴度(231 U/mL)。另外,抗-Nephrin抗體在血清中含量低,普通的ELISA很難準確檢測到如此低豐度的抗體,特別在使用無抗原包被作為陰性對照時,檢測結(jié)果(OD差值)為陰性的概率大,在Hengel等人[36]在新英格蘭雜志的研究中,通過使用對IgG抗體的富集來提高抗-Nephrin抗體在檢測樣品中的豐度。基于“富集路徑”的抗-Nephrin抗體檢測方法研發(fā),是未來高靈敏檢測的一個發(fā)展方向。
(2)是否有其它循環(huán)滲透因子參與pFSGS?已有研究顯示,并非所有被診斷患有pFSGS或MCD的患者的抗-Nephrin抗體檢測都呈陽性。在Watts[37]研究中,在62名MCD患者中,只有18名抗-Nephrin抗體陽性。這一結(jié)果提示,可能有其他循環(huán)滲透因子參與了剩余44例患者的MCD誘發(fā)。在Hengel等人[36]的研究中,觀察到94例特發(fā)性腎病綜合癥患兒為Nephrin抗體陽性,而88例為陰性。在成人MCD患者中有46例抗-Nephrin抗體陽性和59例抗體陰性,pFSGS患者中有7例抗體陽性和67例抗體陰性。這些觀察表明,盡管抗-Nephrin抗體可被視為特發(fā)性腎病綜合征因素包括MCD和FSGS的新的生物標志物和循環(huán)滲透因子,它可能并不是對所有MCD和FSGS患者具有普遍適用性。例如,最近的研究報道,Crb2是一種重要的縱裂膈肌蛋白,敲除足細胞CRB2的小鼠在出生后2個月或出生后立即出現(xiàn)大量蛋白尿,此外還表現(xiàn)出NPHS1&2、PODXL表達減少以及腎小球WT-1細胞減少[55-56]。此外,觀察到給予足細胞CRB2蛋白的小鼠產(chǎn)生針對足細胞蛋白的抗CRB2抗體,伴有蛋白尿和MCD和FSGS[57]的特征性特征。然而,關(guān)于在患有特發(fā)性腎病綜合征的人中檢測CRB2抗體的報道仍然有限。
(3)抗-Nephrin抗體陽性受者腎移植的脫敏治療。脫敏治療方案對抗-Nephrin抗體陽性的受者,術(shù)后FSGS復(fù)發(fā)研究還比較缺乏,目前FSGS復(fù)發(fā)的研究隊列未區(qū)分出抗體陽性這一亞隊列。早期在Gallon等人[9]的報告中,10例高風(fēng)險腎移植受者接受了兩次術(shù)前血漿置換和5次術(shù)后血漿置換,7例患者在終點未發(fā)生FSGS復(fù)發(fā)。在Bressendorff等人[52]最近的一項研究中,一名接受7輪血漿置換治療的患者痊愈,無復(fù)發(fā)跡象,沒有復(fù)發(fā)被認為是聯(lián)合高劑量糖皮質(zhì)激素治療的結(jié)果。Carvajal Abreu,K.等人[58]還指出,在14周內(nèi)進行25次血漿置換,同時使用甲強龍和潑尼松龍,對于預(yù)防移植排斥和FSGS復(fù)發(fā)至關(guān)重要。Kim等人[59]的一項研究還顯示,一名腎移植患者在對鈣調(diào)磷酸酶抑制劑無反應(yīng)后出現(xiàn)復(fù)發(fā),對其進行了13個療程的血漿置換治療,同時給予利妥昔單抗,患者獲得了部分恢復(fù)。也有研究顯示,在66例pFSGS受者的研究中,37例接受血漿置換聯(lián)合或不聯(lián)合抗CD20單抗的受者,有62%有FSGS復(fù)發(fā),27未接受任何脫敏治療的受者中僅有51%復(fù)發(fā)[60]。這些研究表明,預(yù)脫敏方案可降低FSGS的復(fù)發(fā),但目前暫未有大型研究證實,對術(shù)前脫敏治療是否能夠影響FSGS的復(fù)發(fā),還存在不一致的結(jié)果。未來研究脫敏治療對抗-Nephrin抗體陽性FSGS這一亞隊列的脫敏方案顯得比較重要。
(4)最后,目前還沒有研究檢測Nephrin不同表位和抗-Nephrin抗體的相互作用,是否有多個表位表現(xiàn)出抗體結(jié)合能力,及不同表位的結(jié)合能力是否一致等問題并不清楚。對抗-Nephrin抗體結(jié)合Nephrin的特定區(qū)域以誘導(dǎo)Nephrin重新分布和足細胞損傷也不是很清楚。也沒有研究確定阻斷Nephrin表位如何能防止與抗體的相互作用。解決這些問題對于闡明Nephrin抗體致病機制、對開發(fā)針對抗-Nephrin抗體陽性FSGS患者的特異性藥物非常重要。下一步需要對Nephrin不同結(jié)構(gòu)域、可作為抗-Nephrin抗體結(jié)合位點的各種表位以及可阻斷這些表位以抑制與抗-Nephrin抗體結(jié)合的潛在配體進行全面研究。
(本文轉(zhuǎn)自奧根診斷官網(wǎng))
參考文獻
[1]Büscher AK, Konrad M, Nagel M, et al. Mutations in podocyte genes are a rare cause of primary FSGS associated with ESRD in adult patients. Clin Nephrol. 2012;78(1):47-53. doi:10.5414/cn107320
[2]Isaranuwatchai S, Chanakul A, Ittiwut C, Ittiwut R, Srichomthong C, Shotelersuk V, Suphapeetiporn K, Praditpornsilpa K. Pathogenic variant detection rate by whole exome sequencing in Thai patients with biopsy-proven focal segmental glomerulosclerosis. Sci Rep. 2023 Jan 16;13(1):805. doi: 10.1038/s41598-022-26291-y. PMID: 36646731; PMCID: PMC9842604.
[3]Santín S, Bullich G, Tazón-Vega B, et al. Clinical utility of genetic testing in children and adults with steroid-resistant nephrotic syndrome.Clin J Am Soc Nephrol. 2011;6(5):1139-1148. doi:10.2215/CJN.05260610
[4]Gandzali Ngabe, P.E., Bonkano Baoua, D., Lengani, A.H.Y., Yattara, H., Kama Yatte, A., Loumingou, R., Tall, L., Ka, E.F., Niang, A. and Diouf, B. (2023) Focal Segmantal Glomerulosclerosis: Epidemiological, Clinico-Biological, Pathological, Etiological, Therapeutic and Evolutionary Profiles in Dakar. Open Journal of Nephrology, 13, 174-194. doi: 10.4236/ojneph.2023.132017.
[5]Rery TF Yuniarti, Ian Effendi, Zulkhair Ali, Novadian, Suprapti, Elfiani, Novandra AP, Dila Siti Hamidah, Fadil Pramudhya Husein, & Ika Kartika Edi P. (2024). Is It a Tumor or Not? A Case of Focal Segmental Glomerulosclerosis Secondary to Type 2 Diabetes with a Concomitant Renal Pseudotumor.Bioscientia Medicina : Journal of Biomedicine and Translational Research, 8(12), 5801-5813.
[6]Hoyer, J. R., Vernier, R. L., Najarian, J. S., Raij, L., Simmons, R. L., and Michael, A. F., Recurrence of idiopathic nephrotic syndrome after renal transplantation, Lancet, 1972, Vol 2(7773) p. 343-8.
[7]Dantal, J., Baatard, R., Hourmant, M., Cantarovich, D., Buzelin, F., and Soulillou, J. P., Recurrent nephrotic syndrome following renal transplantation in patients with focal glomerulosclerosis: a one-center study of plasma exchange effects, Transplantation, 1991, Vol 52(5) p.
[8]Savin Virginia, J., Sharma, R., Sharma, M., McCarthy Ellen, T., Swan Suzanne, K., Ellis, E., Lovell, H., Warady, B., Gunwar, S., Chonko Arnold, M., Artero, M., and Vincenti, F., Circulating Factor Associated with Increased Glomerular Permeability to Albumin in Recurrent Focal Segmental Glomerulosclerosis, New England Journal of Medicine, 1991, Vol 334(14) p. 878-883.
[9] Gallon, L., Leventhal, J., Skaro, A., Kanwar, Y., and Alvarado, A., Resolution of recurrent focal segmental glomerulosclerosis after retransplantation, N Engl J Med, 2012, Vol 366(17) p. 1648-9.
[10] den Braanker, D. J. W., Maas, R. J., Deegens, J. K., Yanginlar, C., Wetzels, J. F. M., van der Vlag, J., and Nijenhuis, T., Novel in vitro assays to detect circulating permeability factor(s) in idiopathic focal segmental glomerulosclerosis, Nephrology Dialysis Transplantation, 2021, Vol 36(2) p. 247-256.
[11] Gauckler, P., Shin, J. I., Alberici, F., Audard, V., Bruchfeld, A., Busch, M., Cheung, C. K., Crnogorac, M., Delbarba, E., Eller, K., Faguer, S., Galesic, K., Griffin, S., Hru?ková, Z., Jeyabalan, A., Karras, A., King, C., Kohli, H. S., Maas, R., Mayer, G., Moiseev, S., Muto, M., Odler, B., Pepper, R. J., Quintana, L. F., Radhakrishnan, J., Ramachandran, R., Salama, A. D., Segelmark, M., Tesa?, V., Wetzels, J., Willcocks, L., Windpessl, M., Zand, L., Zonozi, R., and Kronbichler, A., Rituximab in adult minimal change disease and focal segmental glomerulosclerosis - What is known and what is still unknown?, Autoimmunity Reviews, 2020, Vol 19(11) p. 102671.
[12] Wei, C., Trachtman, H., Li, J., Dong, C., Friedman, A. L., Gassman, J. J., McMahan, J. L., Radeva, M., Heil, K. M., Trautmann, A., Anarat, A., Emre, S., Ghiggeri, G. M., Ozaltin, F., Haffner, D., Gipson, D. S., Kaskel, F., Fischer, D. C., Schaefer, F., and Reiser, J., Circulating suPAR in two cohorts of primary FSGS, J Am Soc Nephrol, 2012, Vol 23(12) p. 2051-9.
[13] Sharma, M., Zhou, J., Gauchat, J. F., Sharma, R., McCarthy, E. T., Srivastava, T., and Savin, V. J., Janus kinase 2/signal transducer and activator of transcription 3 inhibitors attenuate the effect of cardiotrophin-like cytokine factor 1 and human focal segmental glomerulosclerosis serum on glomerular filtration barrier, Transl Res, 2015, Vol 166(4) p. 384-98.
[14] Doublier, S., Zennaro, C., Musante, L., Spatola, T., Candiano, G., Bruschi, M., Besso, L., Cedrino, M., Carraro, M., Ghiggeri, G. M., Camussi, G., and Lupia, E., Soluble CD40 ligand directly alters glomerular permeability and may act as a circulating permeability factor in FSGS, PLOS ONE, 2017, Vol 12(11) p. e0188045.
[15] Meijers, B., Maas, R. J. H., Sprangers, B., Claes, K., Poesen, R., Bammens, B., Naesens, M., Deegens, J. K. J., Dietrich, R., Storr, M., Wetzels, J. F. M., Evenepoel, P., and Kuypers, D., The soluble urokinase receptor is not a clinical marker for focal segmental glomerulosclerosis, Kidney International, 2014, Vol 85(3) p. 636-640.
[16] Müller-Deile, J., Sarau, G., Kotb, A. M., Jaremenko, C., Rolle-Kampczyk, U. E., Daniel, C., Kalkhof, S., Christiansen, S. H., and Schiffer, M., Novel diagnostic and therapeutic techniques reveal changed metabolic profiles in recurrent focal segmental glomerulosclerosis, Scientific Reports, 2021, Vol 11(1) p. 4577.
[17] Hou, S., Yang, B., Chen, Q., Xu, Y., and Li, H., Potential biomarkers of recurrent FSGS: a review, BMC Nephrology, 2024, Vol 25(1) p. 258.
[18]Wei, C., El Hindi, S., Li, J., Fornoni, A., Goes, N., Sageshima, J., Maiguel, D., Karumanchi, S. A., Yap, H. K., Saleem, M., Zhang, Q., Nikolic, B., Chaudhuri, A., Daftarian, P., Salido, E., Torres, A., Salifu, M., Sarwal, M. M., Schaefer, F., Morath, C., Schwenger, V., Zeier, M., Gupta, V., Roth, D., Rastaldi, M. P., Burke, G., Ruiz, P., and Reiser, J., Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis, Nat Med, 2011, Vol 17(8) p. 952-60.
[19] Chebotareva, N., Vinogradov, A., Cao, V., Gindis, A., Berns, A., Alentov, I., and Sergeeva, N., Serum levels of plasminogen activator urokinase receptor and cardiotrophin-like cytokine factor 1 in patients with nephrotic syndrome, Clin Nephrol, 2022, Vol 97(2) p. 103-110.
[20] da Silva, C. A., Araújo, L. S., Dos Reis Monteiro, M. L. G., de Morais Pereira, L. H., da Silva, M. V., Castellano, L. R. C., Corrêa, R. R. M., Dos Reis, M. A., and Machado, J. R., Evaluation of the Diagnostic Potential of uPAR as a Biomarker in Renal Biopsies of Patients with FSGS, Dis Markers, 2019, Vol 2019 p. 1070495.
[21] Wei, C., M?ller, C. C., Altintas, M. M., Li, J., Schwarz, K., Zacchigna, S., Xie, L., Henger, A., Schmid, H., Rastaldi, M. P., Cowan, P., Kretzler, M., Parrilla, R., Bendayan, M., Gupta, V., Nikolic, B., Kalluri, R., Carmeliet, P., Mundel, P., and Reiser, J., Modification of kidney barrier function by the urokinase receptor, Nat Med, 2008, Vol 14(1) p. 55-63. 10.1038/nm1696.
[22] Meijers, B., Maas, R. J. H., Sprangers, B., Claes, K., Poesen, R., Bammens, B., Naesens, M., Deegens, J. K. J., Dietrich, R., Storr, M., Wetzels, J. F. M., Evenepoel, P., and Kuypers, D., The soluble urokinase receptor is not a clinical marker for focal segmental glomerulosclerosis, Kidney International, 2014, Vol 85(3) p. 636-640.
[23] Maas, R. J. H., Wetzels, J. F. M., and Deegens, J. K. J., Serum-soluble urokinase receptor concentration in primary FSGS, Kidney International, 2012, Vol 81(10) p. 1043-1044.
[24] Maas, R. J. H., Wetzels, J. F. M., and Deegens, J. K. J., Serum suPAR concentrations in patients with focal segmental glomerulosclerosis with end-stage renal disease, Kidney International, 2014, Vol 85(3) p. 711.
[25] Maas, R. J. H., Deegens, J. K. J., and Wetzels, J. F. M., Serum suPAR in patients with FSGS: trash or treasure?, Pediatric Nephrology, 2013, Vol 28(7) p. 1041-1048. 10.1007/s00467-013-2452-5.
[26] Hayek, S. S., Tahhan, A. S., Ko, Y.-A., Alkhoder, A., Zheng, S., Bhimani, R., Hartsfield, J., Kim, J., Wilson, P., Shaw, L., Wei, C., Reiser, J., and Quyyumi, A. A., Soluble Urokinase Plasminogen Activator Receptor Levels and Outcomes in Patients with Heart Failure, Journal of Cardiac Failure, 2023, Vol 29(2) p. 158-167.
[27] Mohammed, M. S. and Ahmed, H. S., Plasminogen activator urokinase receptor as a diagnostic and prognostic biomarker in type 2 diabetic patients with cardiovascular disease, J Cardiovasc Thorac Res, 2023, Vol 15(3) p. 154-160. 10.34172/jcvtr.2023.32895.
[28] Sharma, M., Zhou, J., Gauchat, J. F., Sharma, R., McCarthy, E. T., Srivastava, T., and Savin, V. J., Janus kinase 2/signal transducer and activator of transcription 3 inhibitors attenuate the effect of cardiotrophin-like cytokine factor 1 and human focal segmental glomerulosclerosis serum on glomerular filtration barrier, Transl Res, 2015, Vol 166(4) p. 384-98.
[29] Savin, V. J., Sharma, M., Zhou, J., Gennochi, D., Fields, T., Sharma, R., McCarthy, E. T., Srivastava, T., Domen, J., Tormo, A., and Gauchat, J. F., Renal and Hematological Effects of CLCF-1, a B-Cell-Stimulating Cytokine of the IL-6 Family, J Immunol Res, 2015, Vol 2015 p. 714964.
[30] Savin, V. J., McCarthy, E. T., and Sharma, M., Permeability factors in nephrotic syndrome and focal segmental glomerulosclerosis, Kidney Res Clin Pract, 2012, Vol 31(4) p. 205-13.
[31] McCarthy, E. T., Sharma, M., and Savin, V. J., Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis, Clin J Am Soc Nephrol, 2010, Vol 5(11) p. 2115-21.
[32] Delville, M., Sigdel, T. K., Wei, C., Li, J., Hsieh, S. C., Fornoni, A., Burke, G. W., Bruneval, P., Naesens, M., Jackson, A., Alachkar, N., Canaud, G., Legendre, C., Anglicheau, D., Reiser, J., and Sarwal, M. M., A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation, Sci Transl Med, 2014, Vol 6(256) p. 256ra136.
[33] Komura, K., Fujimoto, M., Matsushita, T., Yanaba, K., Kodera, M., Kawasuji, A., Hasegawa, M., Takehara, K., and Sato, S., Increased serum soluble CD40 levels in patients with systemic sclerosis, J Rheumatol, 2007, Vol 34(2) p. 353-8.
[34] Liu, H., Qi, C. J., Zhuang, Y. M., Gan, J. H., Li, H. L., Yin, C. S., and Zhang, X. G., [Serum levels and clinical significance of soluble CD40 in liver disease], Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi, 2006, Vol 22(6) p. 738-41.
[35] Chebotareva, N., Vinogradov, A., Birukova, Y., Alentov, I., Sergeeva, N., Chemodanova, D., Kononikhin, A. S., and Moiseev, S. V., A pilot study of anti-nephrin antibodies in podocytopaties among adults, Nephrology (Carlton), 2024, Vol 29(2) p. 86-92.
[36] Hengel Felicitas, E., Dehde, S., Lassé, M., Zahner, G., Seifert, L., Schnarre, A., Kretz, O., Demir, F., Pinnschmidt Hans, O., Grahammer, F., Lucas, R., Mehner Lea, M., Zimmermann, T., Billing Anja, M., Oh, J., Mitrotti, A., Pontrelli, P., Debiec, H., Dossier, C., Colucci, M., Emma, F., Smoyer William, E., Weins, A., Schaefer, F., Alachkar, N., Diemert, A., Hogan, J., Hoxha, E., Wiech, T., Rinschen Markus, M., Ronco, P., Vivarelli, M., Gesualdo, L., Tomas Nicola, M., and Huber Tobias, B., Autoantibodies Targeting Nephrin in Podocytopathies, New England Journal of Medicine, 2024, Vol 391(5) p. 422-433.
[37] Watts, A. J. B., Keller, K. H., Lerner, G., Rosales, I., Collins, A. B., Sekulic, M., Waikar, S. S., Chandraker, A., Riella, L. V., Alexander, M. P., Troost, J. P., Chen, J., Fermin, D., Yee, J. L., Sampson, M. G., Beck, L. H., Jr., Henderson, J. M., Greka, A., Rennke, H. G., and Weins, A., Discovery of Autoantibodies Targeting Nephrin in Minimal Change Disease Supports a Novel Autoimmune Etiology, J Am Soc Nephrol, 2022, Vol 33(1) p. 238-252.
[38] Shirai, Y., Miura, K., Ishizuka, K., Ando, T., Kanda, S., Hashimoto, J., Hamasaki, Y., Hotta, K., Ito, N., Honda, K., Tanabe, K., Takano, T., and Hattori, M., A multi-institutional study found a possible role of anti-nephrin antibodies in post-transplant focal segmental glomerulosclerosis recurrence, Kidney Int, 2024, Vol 105(3) p. 608-617.
[39] Norio, R., Heredity in the congenital nephrotic syndrome. A genetic study of 57 finnish FAMILIES WITH A REVIEW OF REPORTED CASES, Ann Paediatr Fenn, 1966, Vol 12 p. Suppl 27:1-94.
[40] Kestil?, M., Lenkkeri, U., M?nnikk?, M., Lamerdin, J., McCready, P., Putaala, H., Ruotsalainen, V., Morita, T., Nissinen, M., Herva, R., Kashtan, C. E., Peltonen, L., Holmberg, C., Olsen, A., and Tryggvason, K., Positionally Cloned Gene for a Novel Glomerular Protein—Nephrin—Is Mutated in Congenital Nephrotic Syndrome, Molecular Cell, 1998, Vol 1(4) p. 575-582.
[41] Kestil?, M., M?nnikk?, M., Holmberg, C., Gyapay, G., Weissenbach, J., Savolainen, E. R., Peltonen, L., and Tryggvason, K., Congenital nephrotic syndrome of the Finnish type maps to the long arm of chromosome 19, Am J Hum Genet, 1994, Vol 54(5) p. 757-64.
[42] Ruotsalainen, V., Ljungberg, P., Wartiovaara, J., Lenkkeri, U., Kestil?, M., Jalanko, H., Holmberg, C., and Tryggvason, K., Nephrin is specifically located at the slit diaphragm of glomerular podocytes, Proc Natl Acad Sci U S A, 1999, Vol 96(14) p. 7962-7.
[43]Topham, P. S., Kawachi, H., Haydar, S. A., Chugh, S., Addona, T. A., Charron, K. B., Holzman, L. B., Shia, M., Shimizu, F., and Salant, D. J., Nephritogenic mAb 5-1-6 is directed at the extracellular domain of rat nephrin, J Clin Invest, 1999, Vol 104(11) p. 1559-66.
[44Holzman, L. B., St John, P. L., Kovari, I. A., Verma, R., Holthofer, H., and Abrahamson, D. R., Nephrin localizes to the slit pore of the glomerular epithelial cell, Kidney Int, 1999, Vol 56(4) p. 1481-91.
[45] Schoeb, D. S., Chernin, G., Heeringa, S. F., Matejas, V., Held, S., Vega-Warner, V., Bockenhauer, D., Vlangos, C. N., Moorani, K. N., Neuhaus, T. J., Kari, J. A., MacDonald, J., Saisawat, P., Ashraf, S., Ovunc, B., Zenker, M., and Hildebrandt, F., Nineteen novel NPHS1 mutations in a worldwide cohort of patients with congenital nephrotic syndrome (CNS), Nephrol Dial Transplant, 2010, Vol 25(9) p. 2970-6.
[46] Denhez, B. and Geraldes, P., "Regulation of Nephrin Phosphorylation in Diabetes and Chronic Kidney Injury", in Protein Reviews: Volume 18, M.Z. Atassi, Editor, Springer Singapore: Singapore. 2017. p. 149-161.
[47] Tian, Y., Chen, X.-m., Liang, X.-m., Wu, X.-b., and Yao, C.-m., SGLT2 inhibitors attenuate nephrin loss and enhance TGF-β1 secretion in type 2 diabetes patients with albuminuria: a randomized clinical trial, Scientific Reports, 2022, Vol 12(1) p. 15695.
[48] Verma, R., Kovari, I., Soofi, A., Nihalani, D., Patrie, K., and Holzman, L. B., Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization, J Clin Invest, 2006, Vol 116(5) p. 1346-59.
[49] Jones, N., Blasutig, I. M., Eremina, V., Ruston, J. M., Bladt, F., Li, H., Huang, H., Larose, L., Li, S. S. C., Takano, T., Quaggin, S. E., and Pawson, T., Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes, Nature, 2006, Vol 440(7085) p. 818-823.
[50] Orikasa, M., Matsui, K., Oite, T., and Shimizu, F., , The Journal of Immunology, 1988, Vol 141(3) p. 807-814.
[51] Kikuchi, H., Kawachi, H., Ito, Y., Matsui, K., Nosaka, H., Saito, A., Orikasa, M., Arakawa, M., and Shimizu, F., Severe proteinuria, sustained for 6 months, induces tubular epithelial cell injury and cell infiltration in rats but not progressive interstitial fibrosis, Nephrology Dialysis Transplantation, 2000, Vol 15(6) p. 799-810.
[52] Bressendorff, I., Nelveg-Kristensen, K. E., Ghasemi, M., Watts, A. J. B., Elversang, J., Keller, K. H., Nielsen, F. C., Szpirt, W., and Weins, A., Antinephrin-Associated Primary Focal Segmental Glomerulosclerosis Successfully Treated With Plasmapheresis, Kidney International Reports, 2024, Vol 9(9) p. 2829-2831.
[53] Cui, Z. and Zhao, M.-h., Anti-nephrin autoantibodies: a paradigm shift in podocytopathies, Nature Reviews Nephrology, 2024, Vol 20(10) p. 639-640.
[54]Batal I, Watts AJB, Gibier JB, Hamroun A, Top I, Provot F, Keller K, Ye X, Fernandez HE, Leal R, Andeen NK, Crew RJ, Dube GK, Vasilescu ER, Ratner LE, Bowman N, Bomback AS, Sanna-Cherchi S, Kiryluk K, Weins A. Pre-transplant anti-nephrin antibodies are specific predictors of recurrent diffuse podocytopathy in the kidney allograft. Kidney Int. 2024 Oct;106(4):749-752. doi: 10.1016/j.kint.2024.07.022.
[55] Tanoue, A., Katayama, K., Ito, Y., Joh, K., Toda, M., Yasuma, T., D’Alessandro-Gabazza, C. N., Kawachi, H., Yan, K., Ito, M., Gabazza, E. C., Tryggvason, K., and Dohi, K., Podocyte-specific Crb2 knockout mice develop focal segmental glomerulosclerosis, Scientific Reports, 2021, Vol 11(1) p. 20556.
[56] M?ller-Kerutt, A., Rodriguez-Gatica, J. E., Wacker, K., Bhatia, R., Siebrasse, J. P., Boon, N., Van Marck, V., Boor, P., Kubitscheck, U., Wijnholds, J., Pavenst?dt, H., and Weide, T., Crumbs2 Is an Essential Slit Diaphragm Protein of the Renal Filtration Barrier, J Am Soc Nephrol, 2021, Vol 32(5) p. 1053-1070.
[57] Hada, I., Shimizu, A., Takematsu, H., Nishibori, Y., Kimura, T., Fukutomi, T., Kudo, A., Ito-Nitta, N., Kiuchi, Z., Patrakka, J., Mikami, N., Leclerc, S., Akimoto, Y., Hirayama, Y., Mori, S., Takano, T., and Yan, K., A Novel Mouse Model of Idiopathic Nephrotic Syndrome Induced by Immunization with the Podocyte Protein Crb2, J Am Soc Nephrol, 2022, Vol 33(11) p. 2008-2025.
[58] Carvajal Abreu, K., Loos, S., Fischer, L., Pape, L., Wiech, T., Kemper, M. J., T?nshoff, B., Oh, J., and Schild, R., Case report: Early onset de novo FSGS in a child after kidney transplantation—a successful treatment, 2023, Vol 11 p.
[59] Kim, Y.-J., Lee, S.-W., Kim, M.-S., Kim, Y.-J., Choi, J.-Y., Cho, J.-H., Kim, C.-D., Kim, Y.-L., Yun, W.-S., Huh, S., Lim, J.-H., and Park, S.-H., Anuria after kidney transplantation diagnosed as early recurrence of focal segmental glomerulosclerosis combined with acute calcineurin inhibitor nephrotoxicity: a case report and literature review, BMC Nephrology, 2024, Vol 25(1) p. 123.
[60]Alasfar S , Matar D , Montgomery RA ,et al. Rituximab and therapeutic plasma exchange in recurrent focal segmental glomerulosclerosis postkidney transplantation[J]. Transplantation, 2018,102(3):e115-e120. DOI: 10.1097/TP.000000000000200
來源: 奧根診斷官網(wǎng)


 科普中國公眾號
科普中國公眾號
 科普中國微博
科普中國微博

 幫助
幫助
 移路相伴
移路相伴 